At micro and nano-scale liquid flow rates, calibration is critical, especially for applications such as volumetric dosing and drug delivery. In particular, for drugs with a very short half-life (in the order of one minute), or for drugs that require a very low blood concentration for toxicity reasons, such as vasoactive or anaesthetic drugs, the exact amount of volume administered as well as the stability of the flow rate are crucial.
OBJECTIVES
Establishment of an infrastructure for calibration of drug delivery systems for flows up to 10-100 nl/min
Development of transfer standards for on-site calibration of drug delivery equipment
Performance evaluation of drug delivery devices, dependence on operating conditions and clinical characteristics
Provision of a good practice guide for drug dispensing and improved calibration services for drug delivery devices
SUMMARY AND RESULTS
Until 2012, however, metrological traceability for these very low flow ranges was only validated in Europe from 16 l/min upwards.
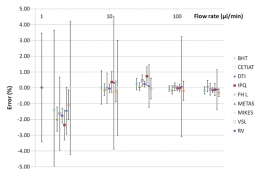
The national metrology laboratories LNE-CETIAT, DTI, IPQ, METAS, and VSL developed primary calibration methods covering a range of liquid flow rates from 10 l/h to 10 nl/min as part of the European metrology research project “HLT07 Metrology for Drug Delivery – MeDD.” These national references have been validated by comparing the measurement results obtained with a Coriolis mass flow meter and a syringe pump (see figure opposite). These results have led to the submission of new calibration possibilities (CMC, Calibration and Measurement Capabilities) that are unprecedented for these flow ranges.
The influence of several physical parameters such as temperature, back pressure, viscosity, and flow pulsations was studied. It was thus demonstrated that Coriolis mass flow meters are less sensitive to the physical parameters studied and therefore constitute transfer standard flow meters suitable for establishing metrological traceability for medical devices.
With regard to infusion devices, several characteristics were tested: start-up time, flow stability, and response time to occlusion, depending on the presence of accessories such as valves, needles, and tubing, and depending on physical parameters such as temperature and liquid viscosity.
The results obtained showed that infusion drug delivery devices are sensitive to conditions of use, particularly at low flow rates and for larger volume syringes. In addition, the start-up time under certain conditions (very low flow rates) can be as long as several tens of minutes.
Throughout this project, the results and knowledge acquired were disseminated to the scientific and medical communities via various media. Initially, a website (www.drugmetrology.com) was created, providing direct and public access to communications related to the project. A workshop organized by the “MeDD” consortium and bringing together members of the scientific and medical communities was held in Utrecht (Netherlands) in May 2015, providing an opportunity to present the results of this project and discuss the implementation of traceable metrological approaches for infusion devices. A guide to good infusion practices was also drafted and made available on the project website.
PUBLICATIONS AND COMMUNICATIONS
BATISTA E., FILIPE E., BISSIG H., PETTER H.T., LUCAS P., OGHEARD F. and NIEMANN A.K., “European research project on microflow measurements – MEDD”, 9th International Symposium on Fluid Flow Measurement, Arlington, United States of America, April 14th-17th 2015.
BISSIG H., PETTER H.T., LUCAS P., BATISTA E., FILIPE E., ALMEIDA N., RIBEIRO L.F., GALA J., MARTINS R., SAVANIER B., OGHEARD F., NIEMANN A.K., LÖTTERS J. and SPARREBOOM W., “Primary standards for measuring flow rates from 100 nl/min to 1 ml/min – gravimetric principle”, Biomedical Engineering / Biomedizinische Technik, 60, 4, 2015, 301–316, DOI: 10.1515/bmt-2014-0145.
DAVID CH., MELVAD C., BISSIG H. and BATISTA E., “Research interlaboratories comparison for small liquid flow rates (2g/h to 600g/h)”, 16th Flow Measurement Conference (FLOMEKO), Paris, France, September 24th-26th 2013.
OGHEARD F., BATISTA E., BISSIG H., PETTER H.T., LUCAS P. and NIEMANN A.K., “Metrological assessment of micro flow-meters and drug delivery devices in the scope of the "MeDD" EMRP project”, 17e Congrès international de métrologie, Paris, France, September 21st-24th 2015, DOI: 10.1051/metrology/20150009004.
LUCAS P., SNIJDER R.A., TIMMERMAN A.M.D.E., BATISTA, E., BISSIG H. and OGHEARD F., “Best Practice Guide”, Version: 13-05-2015.
PARTNERS
- VSL,
- CETIAT,
- CMI,
- DTI,
- IPQ,
- METAS,
- TUBITAK,
- FH Lubeck,
- UMC Utrecht